Seminars
The Research Education Program (REP) presents 25 seminars each year on a variety of topics, designed to inform and assist you through each step of the research process.
With hospital restrictions easing we have now returned to our face-to-face live presentations in the Perth Children's Hospital auditorium. A light lunch is provided for all in-person attendees. Our seminars are also live streamed using MS Teams. Connect directly on your desktop device, or from one of our hosted locations.
All of our seminars are recorded and uploaded within a week from presentation. Find out more about your viewing options.
In 2023 we introduced a set of 4 Research Skills Workshops - presented in-person only. Watch the recordings on our additional resources page. These workshops will be returning again in 2024.
Our Research Skills Seminar and REDCap Workshop Series for 2024 are underway
| Tuesday 30 April |
REDCap Workshop 3: Advanced REDCap and Creating Surveys |
Register |
| Wednesday 1 May | Navigating Research Ethics and Governance in WA Workshop - note new date |
Register |
| Friday 3 May |
Scientific Writing |
Register |
| Friday 17 May | Project Management | Register |
Watch our most recently recorded seminars and workshops
If you were unable to join us for our latest sessions, we invite you to submit our access forms to watch in your own time.
| Friday 19 April | Research Governance | Recording coming soon |
| Friday 22 March |
Introduction to Good Clinical Practice |
Watch the recording |
| Tuesday 12 March | REDCap Workshop 2: Intermediate Walkthrough | Watch the recording |
Join us for our special event to celebrate World Clinical Trials Day!
| Monday 20 May | Setting Up Clinical Trials Workshop | Register |
Library of seminar recordings
Explore our library of seminars to find out more about each seminar topic and its presenter(s).
You can register for an upcoming presentation or access the most recent recording for each topic using the accordion menu below or directly from our Trybooking registration page.
Presented by
Professor Tom Snelling - Director of Health and Clinical Analytics, School of Public Health, University of Sydney and Infectious Diseases Physician, Sydney Children’s Hospital Network
Synopsis
Compared with traditional fixed design clinical trials, adaptive clinical trials have a flexible design that uses accumulating results to make changes to the trial design as it is continues, according to pre-specified rules. This introduces a number of potential advantages including efficiency. This seminar provides an overview of adaptive trials – what they are and why we should consider using them, basic methodology including platform trials and the use of Bayesian statistical methods.
Where to watch
Watch the most recent recording - 14 May 2020
This seminar is not scheduled for re-presentation at this time.
Presented by
Michael Dymock - Biostatistician – Telethon Kids Institute
Visit our presenters page for more information
Synopsis
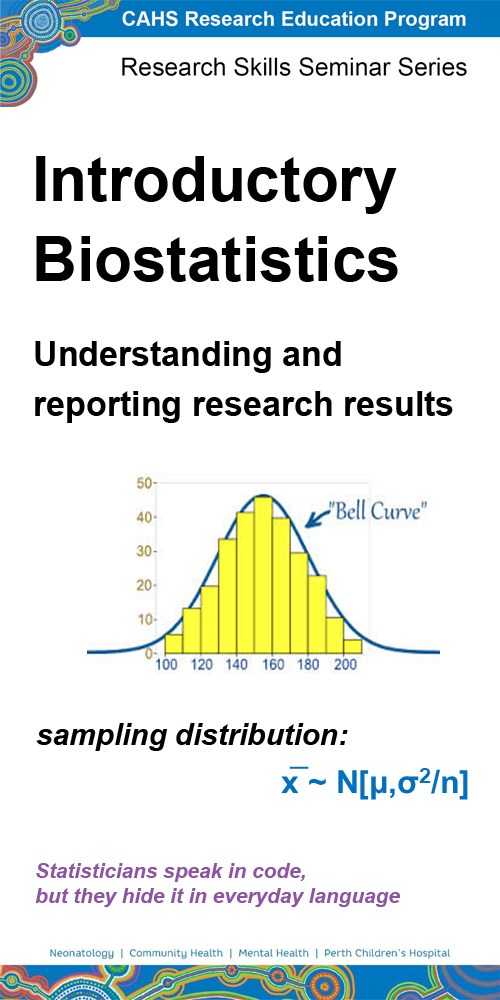
This seminar covers fundamental statistical concepts for clinical researchers, including why we use significance testing, how to interpret confidence intervals and p-values, how sample size and variability affect results, why bias and confounding factors are important considerations in designing studies, and when to seek statistical support.
Where to watch
Watch the most recent recording - 16 February 2024
Presented by
Michael Dymock - Biostatistician, Telethon Kids Institute
Visit our presenters page for more information
Synopsis
This seminar provides an overview of how to perform sample size calculations for different research study designs and demonstrates how to use free "PS" software. It also covers issues specific to clinical trials and assists researchers to identify situations where the best course of action is to consult a statistician.
Where to watch
Watch the most recent recording -30 June 2023.

Presented by
Michael Dymock - Biostatistician – Telethon Kids Institute
Visit our presenters page for more information
Synopsis
To accompany the Rapid Critical Appraisal of Scientific Literature seminar, this seminar tackles critical appraisal from a statistical literacy point of view based on the 2013 Nature paper by Sutherland et al. It uses examples from the medical literature to “help non-scientists interrogate advisers and grasp the limitations of evidence” and will indicate when it is time to consult the statisticians.
These tips are highly relevant for those looking for a refresher in statistical literacy or struggling to understand the seemingly unlimited sources of bias and confounding.
Where to watch
Watch the most recent recording - 27 October 2023

Presented by
Professor Sonya Girdler
Professor of Occupational Therapy
Director | Curtin Autism Research Group
Director Program 3 (Adulthood) | Autism Co-operative Research Centre
Affiliated | Center of Neurodevelopmental Disorders at Karolinska Institute (KIND), Sweden
Visit our presenters page for more information
Synopsis
Systematic reviews play an important role in health research. They provide a high level summary of studies and can inform policy and practice relevant to a particular area of inquiry. Understanding review methodologies is useful for those who wish to undertake a systematic review, or just read one. This seminar provides an overview of several types of reviews, along with simple strategies to focus a review and support review methodology.
Where to watch
Watch the most recent recording - 9 June 2023
 Co-presented by
Co-presented by
- Prof Daniel Fatovich - Emergency Physician, Director of Research East Metropolitan Health Service, Head, Centre for Clinical Research in Emergency Medicine, Harry Perkins Institute of Medical Research, Professor of Emergency Medicine, University of Western Australia
- Mark Woodman - Manager Research Department East Metropolitan Health Service
Visit our presenters page for more information
Synopsis
Effective consent and recruitment strategies are vital for upholding ethical principles of beneficence and justice. This seminar covers important updates to the Guardianship and Administration Act which impact the recruitment of incapacitated adult patients into research. It will also discuss the Waiver of Consent; its requirements for a HREC approval and how it is commonly applied to the recruitment of paediatric research participants.
Where to watch
Register for the next session – 14 June 2024

Presented by
Belinda Frank - Consumer Engagement Manager, Telethon Kids Institute
Visit our presenters page for more information
Synopsis
Every researcher should be actively involving consumer or community members to improve quality and increase impact of their research. Community involvement is increasingly a requirement for funding agencies. This seminar provides a practical introduction and will cover basic principles of consumer and community involvement, the benefits and barriers, and what to put in place to get started.
Where to watch
Watch the most recent recording - 16 June 2023

Presented by
Professor Gary Geelhoed - Executive Director, WA Health Transition Network
Synopsis
This seminar covers COVID-19 research activities and their coordination in WA, including WAHTN's role in funding, coordination and prioritisation.
Where to watch
Watch the most recent recording - 18 May 2020
This seminar is not scheduled for re-presentation at this time.

Presented by
Dr Jane Mugure Githae - CAHS REP Research Fellow
Visit our presenters page for more information
Synopsis
Good data are crucial to a successful project with impact. This seminar explains why we need good data management practices, outlines researcher responsibilities, and provides practical data management planning strategies, including database design and development, data entry, cleaning and storage to ensure the most robust and safe data possible.
Where to watch
Watch the most recent recording - 28 July 2023

Presented by
Dr Nik Zeps - Group Director of Research, Epworth Health Centre, Melbourne VIC
Synopsis
Ethics in research is often perceived as a bureaucratic hurdler to be struggled over.
This seminar will instead cover how to embrace ethics as a powerful tool that can ensure research is relevant and will meet its end point. This seminar will empower researchers to fulfil their obligations more easily, ensuring public trust in their work.
Where to watch
Watch the most recent recording - 5 April 2019
This seminar is not scheduled for re-presentation at this time.

Presented by
Dr Natalie Giles - Manager Research Ethics and Governance, Research Department, CAHS
Visit our presenters page for more information
Synopsis
This seminar reiterates ethical principles and focuses on understanding ethics processes for clinical research and responsibilities for researchers.
It provides practical and up-to-date guidance for completing quality ethics applications.
Where to watch
Watch the most recent seminar recording - 17 November 2023

Presented by
Alexandra Robertson - Director of Research Operations, Research Department, CAHS
Visit our presenters page for more information
Synopsis
Good Clinical Practice (GCP) provides the ethical and scientific standards and guidelines by which all research is conducted, and is a requirement for all researchers to know and apply. This seminar covers key components of GCP including responsibilities, approvals, informed consent, document and data management, and reporting of adverse effects.
Where to watch
Watch the most recent recording - 22 March 2024

Presented by
Dr Tegan McNab - Manager, Grants and Research Development, Telethon Kids Institute
Visit our presenters page for more information
Synopsis
This seminar covers where and how to find grant opportunities, and strategies for putting together a high quality grant application. It also focuses on understanding the grant review process, and how to submit and respond to reviewer comments.
Where to watch
Watch the most recent recording - 20 October 2023

Presented by
Dr Julie Marsh - Senior Research Fellow, Telethon Kids Institute
Visit our presenters page for more information
Synopsis
This seminar covers the common problems investigators face during the pandemic, suggest how solutions can be documented,
and proposes statistical solutions to minimise bias.
Where to watch
Watch the most recent recording - 12 June 2020
This seminar is not scheduled for re-presentation at this time.
Co-presented by
Ashley Schoof - Commercialisation Officer, Telethon Kids Institute
Dr Helen Mikkelsen - Investment Analyst, Brandon Capital Partners
Visit our presenters page for more information
Synopsis
Learn about how to get involved in innovation and commercialisation activities in child and adolescent health in WA. This session will cover: what you need to know and what support is available to help you achieve real-world impact from your research.
Where to watch
Watch the most recent recording - 2 December 2022
Presented by
Cheryl Bridge - Head, Kulunga Aboriginal Unit, Telethon Kids Institute
A/Prof Bep Uink - Dean Indigenous Knowledges, Murdoch University
Shakara Liddelow-Hunt - Research Assistant, Telethon Kids Institute
Visit our presenters page for more information
Synopsis
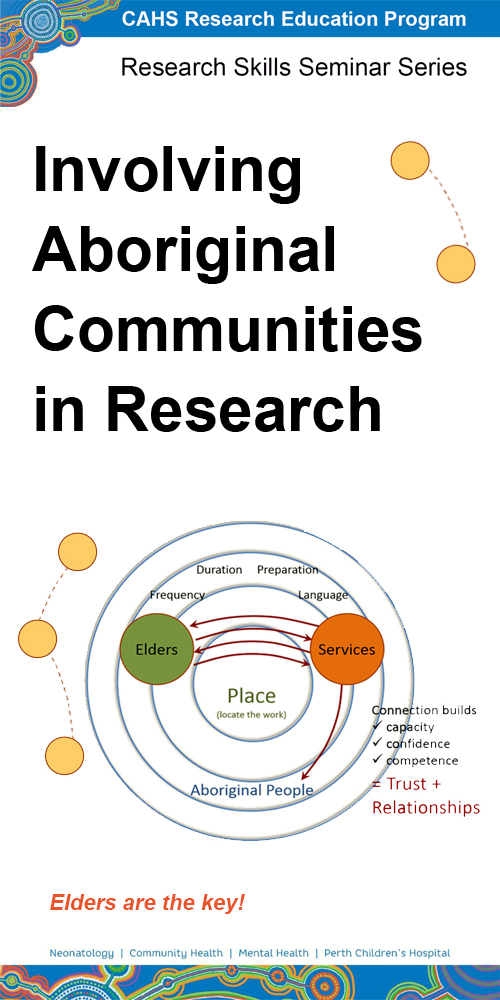
This seminar provides an overview of important considerations for engaging Indigenous people in research, including understanding cultural differences, ethical considerations, and the importance of community consultation.
Where to watch
Watch the most recent recording - 1 September 2023
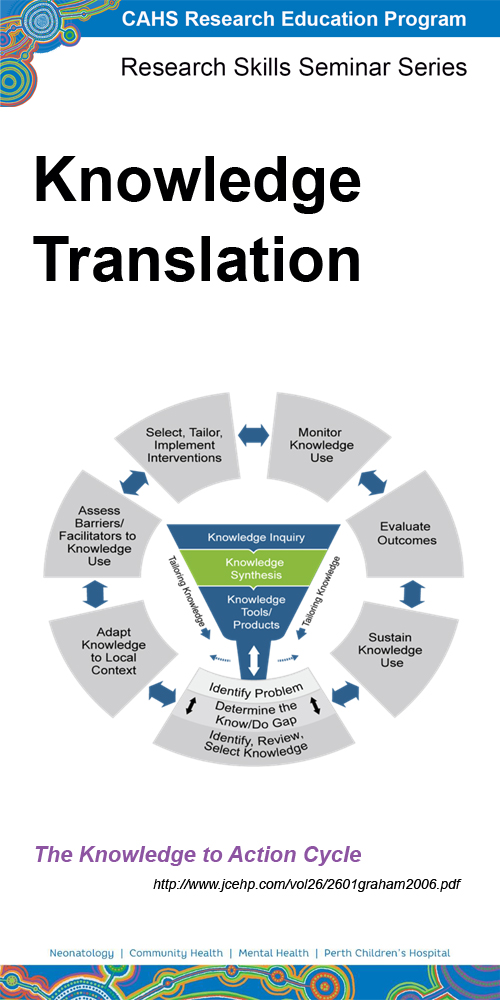
Presented by
A/Prof Fenella Gill - Nursing Research, Perth Children's Hospital and Curtin University
Visit our presenters page for more information
Synopsis
Ensuring that research findings are translated into practice involves a systematic approach from the beginning when you are designing your research. Implementation science bridges the gap between developing and evaluating effective interventions and implementation and de-implementation in routine practice. This seminar covers key elements of implementation research; theoretical approaches, research designs, involvement of stakeholders, behaviour change interventions.
Where to watch
Watch the most recent recording - 8 September 2023

Presented by
Keryn McKinnon, Head Strategic Communications, Telethon Kids Institute
Visit our presenters page for more information
Synopsis
Understanding how to work with the media is essential and a critical responsibility for all researchers, whether it’s the newspaper, TV, radio, or social media. This seminar will provide practical techniques on working with the media and ensuring your bottom line is delivered in an engaging, accurate, and responsible way.
Where to watch
Watch the most recent recording - 18 August 2023

Presented by
Dr Jane Mugure Githae, CAHS REP Research Fellow
Visit our presenters page for more information
Synopsis
Effective presentation of research results is a key component of research translation, a moral responsibility to undertake for your research participants, funders and institution, and an opportunity to get important feedback.
This seminar includes a range of tips on choosing and organising materials, delivery styles and techniques, preparing for questions, gaining confidence, and how to run a session effectively.
Where to watch
Watch the most recent recording - 25 August 2023
 Presented by
Presented by
Melanie Wright - Director of Research, South Metropolitan Health Service
Visit our presenters page for more information
Synopsis
Efficient and effective project management techniques are essential to move your research project from initiation to execution, through to success.
This seminar provides insights to improve internal communications, foster team alignment, facilitate risk management and improve workflows for smooth processes and engaged stakeholders.
Where to watch
Watch the most recent recording - 23 June 2023
Register for the next presentation - 17 May 2024

Presented by
Dr Lorna Davin, Senior Lecturer Medical Education, University of Notre Dame Australia
Visit our presenters page for more information
Synopsis
The use of qualitative research methods is becoming more popular in health either as the primary research method or as part of a mixed methods approach to investigating a health issue. This seminar covers the benefits of using qualitative research; some of the myths associated with the use of qualitative research; the types of qualitative methods; how data is collected and analysed; and how the researcher uses qualitative research to improve health outcomes for individuals, families and communities.
Where to watch
Watch the most recent recording - 24 November 2023
 Presented by
Presented by
Dr Natalie Strobel, Senior Research Fellow,
Centre for improving health services for Aboriginal and Torres Strait Islander children and families,
Kurongkurl Katitjin, Edith Cowan University
Visit our presenters page for more information
Synopsis
Given the sheer volume and variable quality of published papers even in high impact journals, it is essential to have skills to target and rapidly appraise relevant literature to answer current clinical questions. This seminar provides simple strategies to help focus your reading, examine validity of results, and decide whether to accept and apply them in your setting.
Where to watch
Watch the most recent recording - 4 August 2023

Presented by
Dr Jane Mugure Githae - CAHS REP Research Fellow
Visit our presenters page for more information
Synopsis
The Department of Health WA now preferentially supports REDCap use and many health service providers are switching to REDCap as the database of choice for safely entering, storing and reporting on data.
Tips on how to access REDCap, basic REDCap functionality, and where to go for further assistance and resources are also included.
Where to watch
Watch the most recent recording - 5 May 2023

Presented by
Dr Kenneth Lee - Senior Lecturer - Pharmacy Practice, The University of Western Australia
Visit our presenters page for more information.
Synopsis
Research Fundamentals provides a practical introduction to research and examines why we do research, and the steps in the research process. This seminar includes how to decide whether an idea is worth pursuing, through to putting together a quality protocol, and also covers your responsibilities as a researcher.
Where to watch
Watchthe most recent recording - 9 February 2024
 Presented by
Presented by
Dr Natalie Giles - Manager Research Ethics and Governance, Research Department, CAHS
Visit our presenters page for more information
Synopsis
All new research project applications must cover requirements for both ethics and governance. This seminar focuses on the general principles and responsibilities related to research governance, and provides practical tips for preparation of governance applications.
Where to watch
Watch the most recent recording - 13 October 2023
Recording from the 19 April 2024 presentation is coming soon.
 Presented by
Presented by
Dr Tamika Heiden
Founder and Principal, The Research Impact Academy
View our presenters page for more information
Synopsis
There is an increasing requirement for researchers to demonstrate the impacts of their research to funders, stakeholders and to the community.
This seminar will provide insights into the requirements of an impact statement required for an NHMRC Investigator or Synergy grant. You will learn the tools and techniques to develop and write NHMRC impact statements.
Where to watch
Watch the most recent recording - 26 May 2023
Register for the next presentation - 7 June 2024

Presented by
A/Professor Sunalene Devadason, senior researcher at Perth Children’s Hospital and a Graduate Research Coordinator in the UWA Medical School, Division of Paediatrics.
Visit our presenters page for more information
Synopsis
This seminar covers the importance of understanding the rights and responsibilities of both supervisors and supervisees doing research, whether for a formal degree or a small project, and how to get the best out of both roles. It also provides practical tips related to the selection of suitable research projects, supervision frequency and time allocation, different supervision styles, remote supervision and working with multiple supervisors.
Where to watch
Watch the recording from 27 May 2022 (Presented by Professor Jonathan Carapetis AM - Director, Telethon Kids Institute)
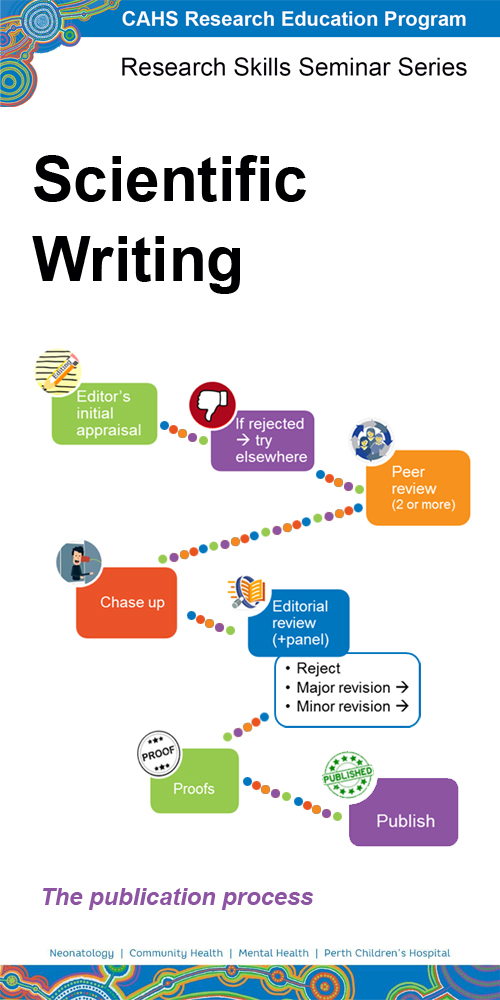
Presented by
A/Prof Tony Kemp - UWA
Visit our presenters page for more information
Synopsis
Writing is the most used channel for communication of ideas, research, and findings. Being able to have quality and effective scientific writing is a fundamental part of successful research translation. This seminar provides a practical overview of scientific writing; including principles of good writing, how to get started, article structure and organisation, how to negotiate authorship, and the publication process.
Where to watch
Watch the most recent recording - 28 April 2023
Register for the next presentation - 3 May 2024

Presented by
Dr Amy Page - Senior Lecturer - School of Allied Health, The University of Western Australia
Visit our presenters page for more information
Synopsis
As a researcher, it is difficult to reach the public and broadcast your work. Building and maintaining your “brand” will help set you apart. This seminar provides the tools to connect with other researchers, build your network, and in the long run, effectively translate your research to a wider audience.
Where to watch
Watch the most recent recording - 8 March 2024
 Presented by
Presented by
Dr Jane Mugure Githae - CAHS REP Research Fellow
Visit our presenters page for more information
Synopsis
Surveys, including clinical audits, are one of the most commonly conducted clinical research projects. There is a lot more to doing these well than meets the eye. This seminar provides practical help for planning and conducting surveys. It includes good survey design, approval pathways, sampling and administration methods, writing high quality questionnaires & data collection instruments, maximising response rates and reducing data errors.
Where to watch
Watch the most recent recording - 2 June 2023


